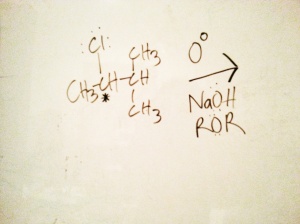orgo
Organic Chemistry: Competing Reactions, Part 4 Quick Guide
Alright, so we are finally to the last installment of the Sn1,Sn2, E1, E2 guide bonanza. From here on in I will refer to the substitutions and eliminations as fundamental reactions collectively. I really wanted to get this entry out before people had finals, hopefully it worked out for you as well. So, let’s get the last entry about fundamental reactions out of the way.
Competing reactions
Eventually the average student will be able to discriminate between the basics of the fundamental reactions. The harder part of the course usually involves being able to discriminate with a fair degree of certainty when a reaction will undergo a Sn1, Sn2, E1 or E2 process. At times the reaction may favor only one mechanism, or it may favor all of them– competing reactions are often why you’ll spend those extra hours in Organic lab purifying the product. Sometimes the reaction is impossible, therefore the answer to the question stem may be the dreaded ‘no reaction’. Interestingly, it takes a remarkable amount of courage and confidence to write ‘no reaction’ on an exam.
Organic Chemistry will be the first course where you are truly inundated with details, i.e. the first time you had to try to drink from a fire hydrant so they say. They key is to work a lot of problems, and keep the details straight. Working a lot of problems will make you familiar with they manner that I will demonstrate to make competing reactions less troublesome.
First, let’s take all the information we already know about fundamental reactions and place it into a form that’s easy on the eyes:
|
|
Reactions |
|||
| Substrate |
Sn1 |
Sn2 | E1 | E2 |
| Me-X |
X |
|||
| 1 |
X |
X |
||
|
2 |
X |
X |
X |
X |
|
3 |
X |
X |
X |
|
Table 1
Some will say Sn1 can also reaction with a primary alkyl halide, but in practice this would quite a monumental feat because primary carbocations are extremely unstable under normal conditions. So, I left it out to avoid confusion.
The table listed above is just an amalgamation of the previous tables I’ve listed so far in the previous guides. From this chart we can state several axioms:
- Me-X alkyl halides are restricted to Sn2 only.
- Primary alkyl halides can undergo all fundamental reactions except E1 and Sn1.
- Secondary alkyl halides can undergo all fundamental reactions.
- Tertiary alkyl halides can undergo all the fundamental reactions except Sn2
In other words, if you get a problem and it’s a Me-X, it’s a slam-dunk, it has to be an Sn2 or NR if the proper reagents aren’t there. Whereas, secondary alkyl halides a multitude of products can be formed all depending on the conditions. So, let’s pretend you had a pesky secondary alkyl halide, such as 2-chloro-3-methylbutane. If we glance at table 1 we are quickly reminded that it can undergo all of the fundamental reactions, given the right conditions.
Selecting by Reagents:
If we put 2-chloro-3-methylbutane with a strong nucleophile we should unequivocally expect an Sn2 and/or E2 reaction. If we put 2-chloro-3-methylbutane with a nucleophile with a solvent (or other conditions) favoring favoring the formation of the carbocation intermediate we should expect to see only Sn1 and/or E1. To summarize what I just said:
| Similar Conditions:-Weak Nu: or base-Polarizing solvent- Rate liming step: carbocation intermediate formation | Similar Conditions:-Strong Nu: or base-Any solvent works, but aprotic solvents best-Rate limiting step: transition state formation | |||
|
Uni-molecular |
Bi-molecular |
|||
| Substrates |
Sn1 |
E1 |
Sn2 |
E2 |
| Me-X |
X |
|||
| 1 |
X |
X |
||
| 2 |
X |
X |
X |
X |
| 3 |
X |
X |
X |
|
table 2, Nu: = nucleophile
Now, if we go back to our 2-chloro-3-methylbutane, we’ve already figured out that if given the same conditions we can assert that the reactions will compete: Sn1 versus E1 or Sn2 versus E2. That is, given the same conditions you should expect either a unimolecular reaction competition (E1 versus Sn1) or a bimolecular competition (E2 versus Sn2).
Sn1 versus E1 Discriminating with Conditions/Reagents:
| Similar Conditions:-Weak Nu: or base-polarizing solvent- Rate liming step: carbocation intermediate formation | ||
|
Uni-molecular |
||
| Substrates |
Sn1 |
E1 |
| Me-X | ||
| 1 | ||
| 2 |
X |
X |
| 3 |
X |
X |
For all intents and purposes, for the first year of Organic Chemistry, it’s best in my opinion to just keep this as a rule of thumb:
When it’s a uni-molecular reactions (Sn1 and E1) expect a mixture products of both Sn1 (watch for rearrangements, and racemic mixtures) and E1.
In practice, selectivity between Sn1 and E1 is poor, as a result lots of products may form, with the most thermodynamic-ally stable product(s) being more abundant. In lab expect to use a lot of fancy lab techniques to isolate the actual product.
Adding heat to the reactants creates a carbocation either way, making the reaction susceptible to both Sn1 and E1. However, as heat is continually put into the reaction the alcohol (substitution) product will tend to dehydrate into the elimination product(s) (alcohols -> alkenes), for this reason in general higher and prolonged temperature biases towards elimination products. Therefore if you started off with 2-chloro-3-methylbutane, reacted it with water and heat, you’d form a mixture of alcohols and alkenes. But, if you left the lab and let the solution boil too long uncontrollably you’d come back only have mostly mostly alkenes. Cold temperatures would shut down both pathways.
Sn2 versus E2: Discrimination with Conditions/Reagents
| Similar Conditions:-Strong Nu: or base-any solvent works, but aprotic solvents best-Rate limiting step: transition state formation | ||
|
Bi-molecular |
||
| Substrates |
Sn2 |
E2 |
| Me-X |
X |
|
| 1 |
X |
X |
| 2 |
X |
X |
| 3 |
X |
|
When it’s a bi-molecular reaction (Sn2 and E2) expect a mixture of products for secondary and primary alkyl halides. However, know that E2 and Sn2 do not work with Me-X and tertiary alkyl halides respectively.
Discriminating between Sn2 and E2 is typically more clear-cut, therefore it’s an extremely testable point. If Sn2 and E2 are competing cold favors Sn2 and heat favors eliminations. You can select for E2 products by using a poor nucleophile but moderate/strong base.
Last Thoughts
If you are just starting Organic Chemistry it’s probably hard to imagine how these four reactions could possibly be important. However, as you gain more experience in synthesizing compounds you’ll soon find the trend to be more obvious. You’ll start the course by looking at alkanes, then perhaps looking at free radical reactions with halogens to create alkyl halides. Pretty much once you’ve loaded your alkane with a halide the sky’s the limit of what you can form given the proper reagents and lab equipment (with proper usage).
Remember, Biochemistry is just Organic chemistry with fancy nomenclature, the better you know Organic the more intuitive everything ‘chemistry’ will seem to be — though in Biochemistry you’re more interested in the results of the reaction than the physics of the mechanism itself.
As always, you can always find me on twitter: https://twitter.com/masterofsleep
Alright folks, back to writing tips about how to apply to medical school Good luck all you cool people with finals! I heart goes out to you as a former premed.
Organic Chemistry: Sn1 & Sn2 Quick Guide, Part Two
So here we are at part two of the Sn1 vs Sn2: Quick-Guide. Here’s a reminder of the layout of these articles:
- The chief goal of this article [the second article] will be show how the chart is one of the few things you’d have to memorize to have general intuition about Organic Chemistry. This is especially true when weighing competing reactions.
- Review Mechanisms of Sn1 versus Sn2, referring to the chart.
The tentative third article would then exist:
- Wrap up substitutions, and finish with mechanisms of eliminations (E1 & E2).
A fourth article might come to add information.
Depending on how comfortable you feel about Orgo, some things will be intuitive. I’m not sure how convincing I’ll be, but Orgo isn’t that bad if you’re methodical. Although this guide will only cover Sn1 and Sn2 my methodology for learning other core mechanisms is similar.
So, let’s just get straight to it. I decided to use 2-chloro-3-methylbutane to form an alcohol, nefariously I have chosen to use a secondary chiral carbon. If we refer to the chart, then it’s blatant that if I had chosen a tertiary alkyl halide, or a Me-X or a primary alkyl halide, then the unequivocal mechanisms would of been Sn1 and Sn2 respectively. However, by picking a secondary halide we are put into a tough situation where we need to rely on our understanding of Orgo because it’s possible to form products with both Sn1 and Sn2, albeit in practice one reaction pathway might be more productive.
So, feast your eyes on the majestic 2-chloro-3-methylbutane [substrate] below. Here’s the typical question stem: In lab you start with 1M of S-2-chloro-3-methylbutane, and .2M of NaOH. Your partner who is in a hurry dumps an additional .5M of NaOH into the solution with your reactants and original concentration of .2M of NaOH. You both note that the reaction precedes more quickly. After purifying your product, find that all of it is now in the R configuration. Predict the products that would form given the reactants and the reagents, also provide the appropriate mechanism.
Depending on how fluent you are in Orgo, this question may seem to have an obvious answer or you’re perhaps experiencing traumatizing flashbacks of the last time you were tested on this type of thing. Now, is the time where I go get coffee while I wait for you to try the first problem. But, before your start, let’s use the following approach to solving all Orgo problems:
- Calm down, it should be solvable, otherwise it wouldn’t of been assigned –unlike in the real world.
- Take the known variables into account, and perform a differential diagnosis to narrow your options before diving deep into the problem.
- Get ‘er down, just start working the problem, staring never gets you anywhere.
- Compare you answer with the things you expected to see in step two, if your experimental data agrees with the theoretical path you originally presumed then there’s a good chance you’ve go the answer.
- Confirm, then do some accounting.
So, you’ve tried the problem yourself first right?
Okay, let’s discuss how I quickly run through the things in the table provided to quickly discriminate between Sn1 and Sn2 to take care of the problem. All I need to do is weigh the evidence for either mechanism, the mechanism with the most evidence is the winner.
Sn1 versus Sn2: Which Exhibits the Greater Preponderance of Evidence?
I could of chosen to start to weigh the evidence at any point of the chart, but I have purposely chosen to start with focusing on the substrate (reactants).
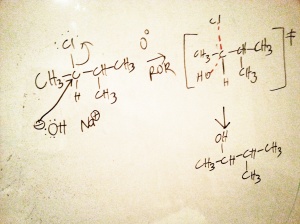
After verifying it as a worthwhile leaving group, I’d classify the substrate first (3, 2, 1, Me-X etc.), it may help narrow down if it’s going to be Sn1 or Sn2. Recalling that I chose a secondary alkyl halide, I won’t get off the hook that easily because the reaction can be either Sn1 or Sn2 — in practice it’s always worth checking. Next, let’s look at the conditions: the reactants are immersed in an ether solvent (ROR), and the temperature of the solution is at 0 degrees Celsius. Ethers promote Sn2 reactions, while doing nothing to help Sn1 reactions because they do not help encourage stable ionization of the alkyl halide. Furthermore, the low temperature does little to provide the activation energy needed to ionize the alkyl halide necessary to initiate a Sn1 reaction. Looking at the nucleophile candidate, the hydroxide ion, all we need to do is recognize that it’s a moderately strong nucleophile (and a moderate base later when we discuss eliminations). Typically strong nucleophiles are seen in Sn2 reactions while weak nucleophiles are used in Sn1, even more evidence for a Sn2 reaction. This should make sense if we consider that the first step in Sn1 is to encourage ionizing of the reactant, a strong nucleophile would try to steal the glory before the alkyl halide had a chance to ionize, i.e. try to act as an Sn2 nucleophile if conditions permitted, even more Sn2 evidence. Next, there’s that bit about the varying concentrations of sodium hydroxide and the reaction rate. If we recall that Sn2 reactions can proceed more quickly if we vary the substrate or the reagent (aka Sn2 = bimolecular nucleophilic substitution), this sort of puts the nail in the coffin, giving us more than enough evidence to assert that it’s going to provide via Sn2. And, for the overkill note that all of the product had a 100% configuration switch, and only Sn2 does that.
So, the correct mechanism should show a Sn2 mechanism, and all the pertinent details hallmark to a Sn2 mechanism e.g. showing the transition state. The general answer of the Sn2 pathway is below, however please note that I didn’t include the best answer because I didn’t show the mechanism in proper stereochemistry terms (that’s for you to enjoy).
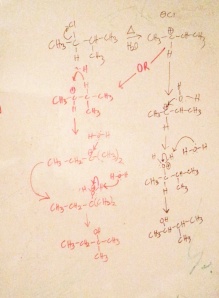
Now, let’s use the same substrate again, 2-chloro-3-methylbutane to do an Sn1 pathway. Here’s another fake question stem: Your lab partner puts 2-chloro-3-methylbutane in a test-tube with water and prepares for distillation. After 45 minutes you’re finished and purify the product, you partner then runs the sample through a NMR machine. You find that the product solution has both a secondary alcohol and a tertiary alcohol. Provide the mechanism to explain this solution, and predict the products. Assume there is no contamination of the sample.
[It’s your turn to draw 2-chloro-3-methylbutane with water]
To be consistent, I’ll run down the list on the table to show that all of those little things you learned in Orgo still apply. First off, the leaving group is a proper one, so it’s not a waste of time to attempt this problem. We know that it’s secondary, so classifying the substrate only confirms to us that Sn1 is a viable idea. What about the solvent? While the solvent is this case is the water. We all know that water allows for salts to dissolve in it, so by nature water encourages ionization, sounds like a Sn1. Let’s also note that the temperature is boiling, this is likely done to input energy to overcome the activation energy, smells like a Sn1 reaction. And if we consider the weak nucleophile, water, we just simply need to recall that Sn2 requires a good nucleophile, so process of elimination leads us to Sn1; it sure tastes like a Sn1 reaction doesn’t it? Lastly, the mixture of products should of been a dead give away, because Sn1 mechanisms allow for hydrogen/methyl shifts.
I’d like to take this time to remind you that the same product is formed using Sn1 and Sn2. Sn2 formed the product, but only the inverted form. So if you wanted to have retention of the configuration you’d have to use Sn1, but then you’d have to deal with having multiple products form, complicating the purification of the final product.
Anyways, I hope this Sn1 and Sn2 guide wasn’t too confusing, and hopefully now you feel better when it comes time to discriminate between Sn1 and Sn2 reactions. Look out for the next entry (part 3 of 3, hopefully) in this series, where we will quickly go over E1 and E2, and truly discuss competing reactions.
As always, you can find me on twitter https://twitter.com/masterofsleep
Future changes to this post:
Will go back and redo examples with a scanner, I had to use my whiteboard in my room — yes, I have a whiteboard in my room.
I will probably append this article with the chart I keep talking about from part 1 to make this post easier to read.
Search and destroy for errata.
Organic Chemistry: Sn1 & Sn2 Quick Guide
Hello!
I’m putting this piece together for a few readers who have finals coming up, I felt their a pain and decided to try to help them from afar as a former Organic Chemistry tutor — as a former premed, I feel their pain. This article will be part 1 of perhaps 2 or maximum 3 articles related to Sn1/Sn2 & E1/E2. So, if you don’t particularly care about Organic right now, you can put this article away later, print it out and put it on your coffee table to impress your friends with chemistry small talk.
The article will only be in the following format:
- Introduction about my own Organic Chemistry situation when I was a student, to put my situation into context with yours.
- Brief prelude into Sn1 and Sn2, the big picture + quick reference chart that should be used in conjunction with the articles 2/3 to come.
The second article will be the following format (tentative):
- The chief goal of this article will be show how the chart is one of the few things you’d have to memorize to have general intuition about Organic Chemistry. This is especially true when weighing competing reactions.
- Review Mechanisms of Sn1 versus Sn2, referring to the chart.
The tentative third article would then exist:
- Wrap up substitutions, and finish with mechanisms of eliminations (E1 & E2).
A fourth article might come to add information.
Introduction: Orgo Hard but Doable
Organic Chemistry (or more affectionately, Orgo) is probably the leading cause of night terrors among premedical students — premeds have even been known to develop a spontaneous stressed induced eye-twitch around finals. I was a nontraditional premed, and my major didn’t require a year of Orgo, so taking the Orgo series were electives where I received grades B+, A, A, for each quarter respectively. My school was a Polytech university, so the class size was pretty small, there were only about 30 students in the 1st quarter, and it went down to about 20+ for the last quarter. Towards the end of the last quarter however only about 10 people showed up for the final. This allowed for the professor to make a menacing scowl at you each time you failed an exam or quiz, this was often a large motivator to do better — or assume the fetal position. Despite the positive of having a small class size, the course is frequently retaken 2-3 times (it’s not uncommon for our 1 year sequence to turn into 2 years just to pull C’s).
I’m sure it’s a conspiracy, but life seems to get more complicated when you take Orgo. This is because you’re probably towards the home-stretch in your premed career, and you’re likely to have more on your plate then any other time previously as a consequence. This is especially true if you are a nontraditional. When I was taking Orgo, I had to really work on my premed experiences to catch up with traditional premeds. As a consequence, at any given time during the Orgo sequence I was carrying 14-21 units, and conducting undergraduate research, and working, and still scheduled time for my friends, social drinking, and family. Furthermore, I have a terrible memory, so I knew heavy memorization wasn’t going to do it for me, but sometimes it’s a necessary evil. So, Orgo is doable without becoming an Orgo ogre (I apologize, I couldn’t help myself).
Without further adieu long and behold the Sn1 versus Sn2 table before you.
The reason why first year Orgo texts introduce substitutions early on is because a lot of reactions end up just being substitutions (or eliminations). The gist of Sn1 and Sn2 are quite straight forward, you start with something (substrate) and you want to take a nucleophile and stick onto your substrate, and in the process of making your product you kick a group off called the leaving group (because it gets kicked off).
That’s actually all there is to it, the tricky party is figuring out when it’s an Sn1 or Sn2, or the dreaded no reaction. In order to become proficient I first took the time to be practice my fluency in the rules of two the two reactions. If you look at the chart that I compiled for us, then you’ll see that after careful introspection, the two reactions are really nothing alike except that they prefer to have good leaving groups.
Before we go on, I encourage you to print this chart, and keep a copy for yourself so you can refer to it even for your real assignments. If you can recreate it from scratch, then you’re on your way to going above and beyond the call of duty.
This concludes the first article, more to come soon.
terms NR = no reaction, RXN = reaction. Organic Chemistry has a lot of short hand, scientists are lazy.
Table
|
Things to Consider |
Sn1 |
Sn2 |
| Substrate | In general the more substituents the better; priority of RXN 3 >2 >, 1 & Me-X is for all intents and purposes will yield NR | In general the less substituents the better; priority of RXN Me-X > 1 > 2, NR with 3 |
| Kinetics | 1st order kR[substrate]; kinetics depends on only the concentration of the substrate | 2nd order kR[substrate][nucleophile], i.e. the kinetics depend on both the concentrations of the substrate and nucleophile |
| Nucleophile | Weak nucleophiles work just fine(e.g.CH3CH2OH, H2O) | Strong nucleophiles (e.g. NaOH) |
| Substrate’s leaving group (LG)* |
Good leaving groups only
|
|
|
Solvents + Other Stuff in Solution |
||
| Yet Even More Stuff to Consider |
Sn1 |
Sn2 |
| Solvent | Solvents should encourage ionization | A lot of solvents work* |
| Misc. Reagents | Typically when you see transitions metals tossed in (e.g. AgNO3) chances are its likely encouraging ionization, and it’s a Sn1 | No gimmicks required usually |
| Temperature | Heat encourages the reaction | Typically excess heat discourages the reaction |
| Stereochemistry | Mixture of products, S and R inversions, retentions, and rearrangements | The nature of the reaction requires a 100% inversion of the chiral configuration from substrate to product, i.e. always inverted S -> R or R -> S; rearrangements are impossible |
As usual, just ask me something or other on twitter! https://twitter.com/masterofsleep
Updates This Week
Thanks for reading!
Thanks for all of the support, kind words, questions, and interesting conversations. Remembering that the point of this blog was to help nontraditional premeds (don’t worry traditional premeds, I love you too) I’m going to be dedicating a few blog entries to people who made personal requests. When I first made this blog I half expected no one to read it, so thanks for helping me get over 1000 views within the first week or so after launching.
So, my tentative blogging schedule is:
This week -11/23 – 11/24, Organic Chemistry tips to learn basic mechanisms for substitutions and eliminations.
Next week – back to AMCAS stuff and answering another reader with a blog entry. If you have any requests just message me, or tweet me at twitter (you don’t need to follow me to get an answer) https://twitter.com/masterofsleep.
My Personal Medical School App Life
I hear back from a medical school in Chicago perhaps this week. It was my 3rd interview in a string of interviews I did in one week, after a multi-flight tour of the US, it took me seven flights to finally interview in Boston, Michigan, and finally in Chicago. Needless to say, exhaustion did take its toll. It was consisted of two interviews, one was a physician it was a great “bad cop, bad cop” session. The second interview was with a medical student on their way to graduation, that was a very fun interview.
Medical student interviewer: “What could pick a super power what would it be?”
Me: “…the ability to fly, that would be pretty awesome.
Medical student interviewer: “Now here’s the kicker, how would you use that power to help others?”
Me: “…I’d do a mobile organ transplant delivery service. Hmm..I’m sure glad I didn’t pick laser eyes for my super power”.
As you can probably tell, I have no problem quacking jokes during my interview, if they school doesn’t get my humor than I know it’s not my fit for the next 4 years. So, with that split, I have no idea if I’m getting rejected or not (I’m leaning towards wait-list), but I’ll live either way =).
Organic Chemistry: Nontraditional Premed into Organic Tutor Part 1
Organic Chemistry aka: Orgo, Ochem, Carbon Chemistry, the Widow-maker 
Want to see a premed have a cardiac arrest? Just tell them their Organic midterm just got moved up a week — though you’d have to be dastardly fiend.
If you haven’t had to sat for an exam for Organic then there’s no real way to capture But, there’s a type of solidarity that comes out of course. Everyone takes it. From the fledgling premed to the grand physician we all share that post traumatic stress of taking the year of Organic Chemistry. Most of the rumors are true, Organic Chemistry is is probably the hardest course most premeds have ever taken up until that point. You’ll sit there lecture, take decent notes and swear you got it, go home study study over the weekend only later to be molested by the final.
Over the progression of the year, those people who text and nap during class disappear, and it’s only you and the survivors braving the storm in the end. But, those who make it out usually build great relationships, perhaps through shared PTSD or survivors guilt. I’m not a chemistry major, and I’m not a traditional premed either, but I got through it with an A average, and was eventually hired as a department recommended Organic Chemistry tutor at my university. Though, I was once trounced by the Orgo monster to for a brief spell, in fact at after receiving a D on my first quiz I was sure my life was over. But, I duct-taped my ego back together and conquered the course. Organic Chemistry is a character building series, I learned what ‘studying’ meant, this had a direct translation to my MCAT score. The most important lesson that we all gain from the course is to experience what it feels like to ‘try to drink from a fire-hose’. (Random thought: for some reason scientists are obsessed with hoses and/or water analogies)
Now, I’ve tutored the course for a couple of years, including labs, I’ll just pass on stuff that did and didn’t work for myself and my students who later went on to pass Organic Chemistry with flying colors.
My Basic Study Pattern for Organic Chemistry:
- Know my enemy.
- Prep before class
- Attend class, take notes.
- Summarize pertinent chapter sections, do homework 3×4 times, review past mistakes.
- Attend office hours after I’ve exhausted myself trying by myself.
- Reconcile everything and prep for next class.
Originally this post started off as one composition, but that was a little over ambitious. So, I just decided to release it in parts otherwise I’d be stuck in editing purgatory forever. So, this blog posting will just cover how to prep before class, and the rest will roll out later.
Know your enemy.
It’s important to know your course time line, so have several copies of your syllabus, keep one on your phone as a PDF to always have one with you. Use your syllabus to guide your reading and priorities.
Syllabus: see the trees and the forest, and perhaps the occasional leaf. 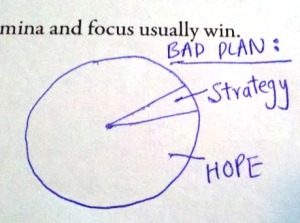
If you are any thing like me, then Organic is probably the first time you actually paid attention to the syllabus. But, its really important to have a clear idea of the expected schedule and grading policies. Students who don’t know their syllabus will often don’t know how to allocate their effort, waste time on low yield material while often missing the bigger picture. I’m not neurotic enough to schedule my whole year out, but I have found it worth the time to refer to the syllabus to see if your ‘mastery of the material’ is on course with the schedule. It’s easy to feel overwhelmed in Organic, so it’s important to stop and take perspective sometimes, knowing your syllabus allows for you to ‘see the forest‘.
Know Your Organic Professor Colleague Unknowingly to you, the chemistry department will have a set of standards to be taught across the board to all students. However, it as at professors’ discretion in how about to dispense lessons and evaluate your understanding. Professors aren’t taught to teach, so often they’ll rely on what they’re accustomed to: their guiding principles learnt during their post doctoral work or recent industry experience etc. For example, if you have a professor that likes kinetics then you’ll probably see a lot more problems with Ka values or perhaps thermodynamic stability product problems. While other a synthetic chemist might be more concerned with your understanding of ‘bread and butter’ mechanisms; and would be expected to know a lot of reactions off the top of your head. Maybe your professor runs the NMR machine, in that case they’re probably a sadist, good luck.
You ought to know your professor well. In fact, it’s best if you move past the typical professor/student relations, that is waiting to be fed. Organic Chemistry is just too difficult and broad to expect to have all of your intimate problems addressed during class by you just raising your hand. Even after class the professor tends to either have to leave go make space for another course, attend their office hours, or do fancy chemistry stuff; so don’t be surprised if your professor is curt with you if after week 8 you’re note quite sure what an orbital is. The easiest way to address this is to pay thousands of dollars to hire an expert to address your questions individually a few times a week. Hey, you already are paying for that, it’s called office hours! If you have any personal qualms with your professor, you’ll have to learn how to have a productive relationship. Your personal relationship with your professor will get no play from medical school admissions committee — just like later your attending treating you like dirt isn’t a valid excuse for you failing boards. To get the most out of the relationship, don’t be intimated by their laurels, think of them as a respected colleague as opposed to chemistry pope.
Vocabulary: You must speak the language
Organic has it’s own lingo, it’s own niche verbiage, at first it may seem asinine but eventually you’ll find it essential. If you don’t become well versed in the vocabulary than you might of as well of accidentally attended a Japanese language course when the professor is lecturing or explaining things to you in office hours. Also, Organic Chemistry is infamous for having “ambiguous question stems” for midterms or finals, and guess what your professor usually won’t give any “hints” to what the question means. Good news everyone, there are actually rarely any ambiguous material on a real Organic exam. Instead students typically have a poor understanding of the real yet subtle differences between two terms, a large part of the test is based on whether or not you understand the question stem. And on the rare occasion that a question is truly ambiguous, professors are usually so disappointed with themselves they might give points to everyone always, this adjusts everyone’s final grade. To get the most out of office hours you and your professor must be speaking the same language. So, with that in mind, develop an Organic accent as soon as possible.
Stay tuned for the upcoming entry about prepping for class and how to make the best of your class time. As always, feel free to contact or follow me at https://twitter.com/doctorORbust